InOut Biome, LLC Gut microbiome sampling
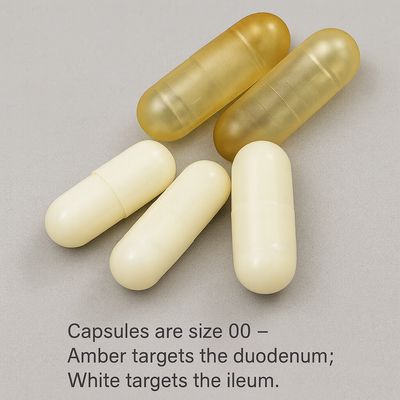
Ingestible Capsule
Mechanical - Single Use
Most microbiome studies rely on fecal samples—even though these reflect only the final stage of digestion. Many microbial communities, metabolites, enzymes, and molecular signals active in the upper gut do not survive the transition to stool.
InOut Biome™ is developing an ingestible sampling capsule for research use in humans and animals. The goal is to provide practical access to localized intestinal environments—capturing molecular signatures directly at the site of activity.
Important biological processes occur upstream of the colon, including:
• Microbial co-occurrence and functional interactions
• pH-sensitive and oxygen-sensitive pathways
• Enzyme activity and metabolic transformation
• Region-specific microbial populations
• Early biochemical events not reflected in fecal material
These signals are often obscured or lost before reaching stool. Localized sampling allows them to be studied more directly.
Despite substantial interest and investment across the field, reliable localized intestinal sampling with molecular stabilization remains technically challenging and largely unavailable for routine research.
The capsule is an early-stage research platform designed to support scientific investigations of localized intestinal content.
Capsule features include:
• Targeted uptake of site-specific intestinal fluid
• Immediate activation of stabilization* chemistry
• Maintain anaerobic internal environment
• Compact mechanical design suitable for ingestible research formats
• Modular adaptation for human or animal use
• A potential 7-day sample stability window, pending further study
Current development focuses on sampling behavior, internal conditions, and compatibility with sequencing and metabolomics workflows.
*"Stabilization refers to suppressing post-capture biological and chemical change while retaining the native molecular and microbial structure present at the moment of sampling.”
• Bacteria and archaea
• Virome and fungal components
• Functional gene pathways
• Microbial co-occurrence patterns
Sequencing Compatibility:
• Shotgun metagenomics
• NGS (next-generation sequencing)
• Virome and fungal sequencing
• 16S rRNA sequencing
Compatible with:
• LC-MS (liquid chromatography–mass spectrometry)
• GC-MS (gas chromatography–mass spectrometry)
• NMR (nuclear magnetic resonance spectroscopy)
• Enzymes and intermediates
• Peptides and small molecules
• pH-sensitive and redox-sensitive environments
Performance studies are ongoing.
The platform is being developed to support studies of:
• Small intestine and upper GI biology
• Metabolite and enzyme dynamics
• Microbial pathways and networks
• Regional differences along the gut
• Sequencing-based microbiome and functional gene analysis
Research use is supported for:
• Dogs
• Swine
• Other mammals
Applications may include nutrition, digestive biology, host–microbe interactions, and multi-omics investigations.
Areas of ongoing development include:
• Sampling performance
• Molecular stabilization behavior
• Sequencing and metabolomics compatibility
• Cross-species study design
• Pilot-scale and exploratory studies
The technology is not FDA approved and is available for research use only.
Researchers interested in potential applications within their projects are welcome to connect.
InOut Biome holds issued and pending patents.
Groups interested in research partnerships, developmental collaboration, or business engagement are invited to contact us.

